- The pH scale ranges from 1 (very acidic) to 14 (very basic or alkaline). Substances with a pH of 7 are neutral.
- pH is a chemical property of a substance.
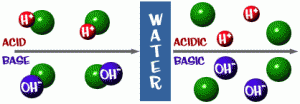
- Simply speaking, acids release (H+) ions (positively or negatively charged particles) when placed in an aqueous (water-based) solution, while bases release (OH-) ions. More specifically, an acid is a molecule that can donate an electron pair to a nearby molecule, while a base is a molecule that can accept an electron pair from a nearby molecule.
- Pure water is neutral. Water molecules exist in equilibrium, or balance, with hydrogen (H+) ions and hydroxide (OH-) ions. If acid is added to water, the H+ concentration increases. If base is added to water, the OH- concentration increases.
H2O -> H+ + OH–
- The pH scale is a measure of the number of H+ ions in a solution. The more H+ ions generated by the molecule when in an aqueous solution, the more acidic it is, and the lower the pH. Conversely, the fewer H+ ions, the more basic, and the higher the pH.
- Substances with an extreme (very high or very low) pH can be dangerous because the high concentration of ions makes them very reactive.

The pH scale ranges from 0-14. Substances with a pH of 0 are very acidic, while substances with a pH of 14 are very basic.
Here are some common substances and their pH levels:
1.8 – 2 lime juice
2.4- 3.4 vinegar
2.9- 3.3 apple juice
3.5- 4.5 grapes
5.2 acid rain
5.7 normal rain
6.5 – 8 drinking water
7.35 – 7.45 human blood
7.36 – 8.21 sea water
8.4 baking soda
11.5 household ammonia
12.5 household bleach
- The pH scale is logarithmic, so a substance with a pH of 7 is ten times more acidic than a substance with a pH of 8. For example, if lime juice has a pH of 2 and vinegar has a pH of 3, lime juice is 10 times more acidic than vinegar.
- Solutions can be classified into five broad categories:
Strong Acid: An acid that has a very low pH (0-4).
Strong Base: A base that has a very high pH (10-14).
Weak Acid: An acid that only partially ionizes in an aqueous solution. That means not every molecule breaks apart. They usually have a pH close to 7 (3-6).
Weak Base: A base that only partially ionizes in an aqueous solution. That means not every molecule breaks apart. They usually have a pH close to 7 (8-10).
Neutral solution: pH is very close to 7.
- The ocean’s pH varies by location, depth, and temperature, but is usually between 8.1 and 8.2.
- Marine organisms are adapted to survive at very specific pH levels. When the pH level of the ocean in a certain area rises or falls, organisms are usually negatively affected by the change.
Review Questions
- Define pH.
- Explain the difference between an acid and a base.
- Why is the pH scale a logarithmic scale?
- What is the normal pH of the ocean? Why is it important for ocean pH to remain steady?
Glossary
Acid: A substance that increases the H+ concentration when added to an aqueous solution
Aqueous Solution: A solution in water
Base: A substance that increases the OH- concentration when added to an aqueous solution
Equilibrium: A stable situation in which two forces are in balance
Ion: a charged particle
Logarithmic: A scale that is based on the power to which each number must be raised in order to produce a given number
pH: A measure of how acidic or basic a substance is
pH scale: A measure of the number of H+ ions in a solution
Strong Acid: An acid that has a very low pH (0-4).
Strong Base: A base that has a very high pH (10-14).
Weak Acid: An acid that only partially ionizes in an aqueous solution. That means not every molecule breaks apart. They usually have a pH close to 7 (3-6).
Weak Base: A base that only partially ionizes in an aqueous solution. That means not every molecule breaks apart. They usually have a pH close to 7 (8-10).